Patients who suffered debilitating immune reactions after being implanted with metal-containing devices have joined doctors, scientists and industry representatives to testify before a United States government advisory panel probing the risks of immunological responses to metals placed inside the body.
The hearing represented the most systematic look by the U.S. Food and Drug Administration at the issue of adverse reactions to metals, a problem that affects a minority of implant patients but one that can cause severe pain, neurological damage and cognitive impairments.
“I’m in a great deal of pain, so please bear with me,” testified Sue Francis, a hip implant patient who has experienced severe health effects stemming from her reaction to metal. “We need to recognize that these metals from day one are interacting with our bodies.”
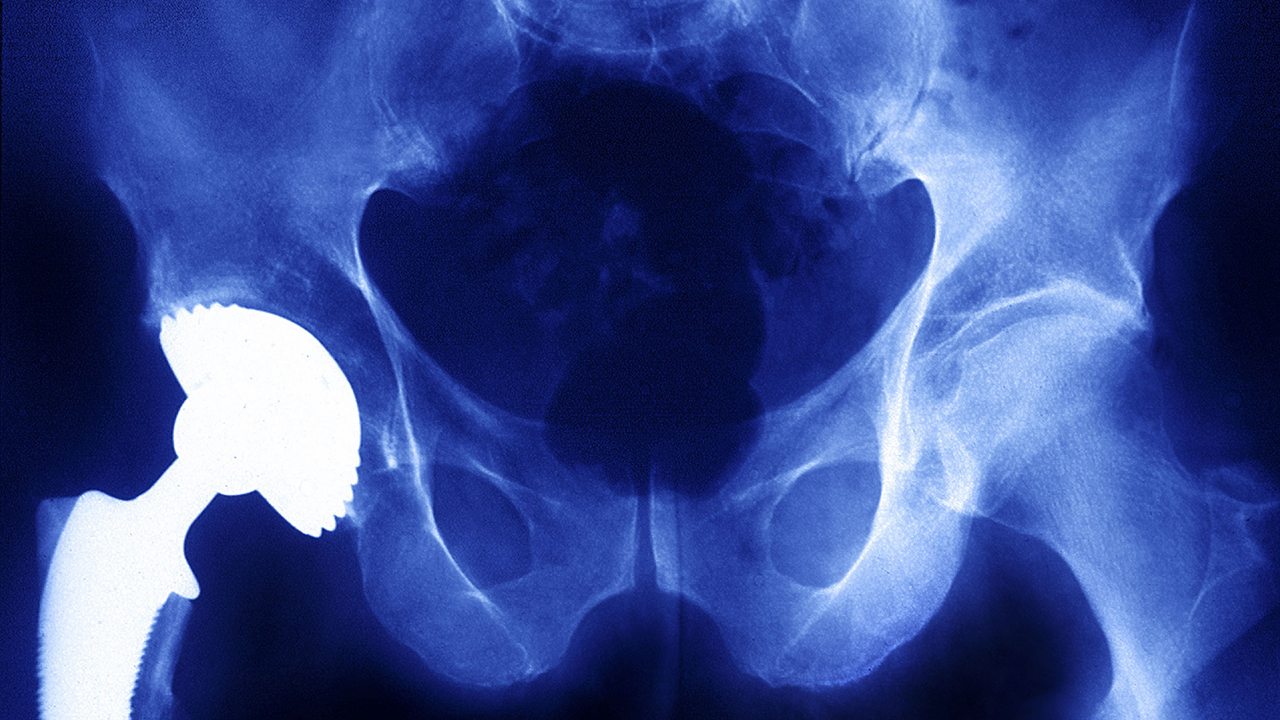
Metals are a major component of common devices such as artificial hips, spinal fusion implants and the contraceptive coil Essure, but there is limited scientific research on auto-immune and allergic responses to them.
The International Consortium of Investigative Journalists reported extensively on hip implants and Essure as part of its global Implant Files investigation, which revealed massive gaps in medical device oversight that left patients vulnerable to flawed and poorly tested products.
Patients and their advocates urged the FDA to order more detailed disclosure of the metals used in devices, to require device labels to disclose the risks associated with metals, and to send a letter to doctors across the country alerting them to the threat of adverse metal reactions.
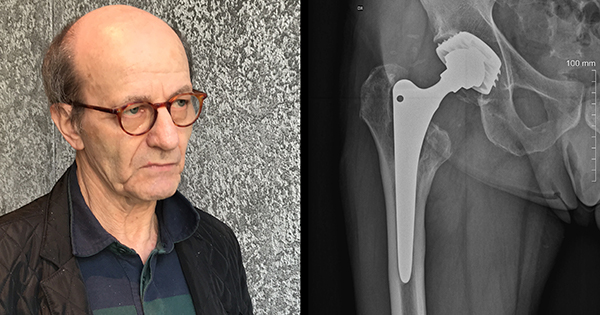
Some of the most forceful testimony came from Dr. Stephen Tower, an orthopedic surgeon who suffered severe cobalt poisoning from his own hip implant, and has since dedicated himself to researching metal poisoning by hip implants.
This is not a metal-on-metal problem. We are seeing this with many popular hip designs – orthopedic surgeon Stephen Tower
Tower said the threat was not limited to metal-on-metal hip implants, which have been the subject of massive jury verdicts and recalls, and are now widely understood to pose serious health risks.
“This is not a metal-on-metal problem,” Tower said. “We are seeing this with many popular hip designs that are still commonly implanted.”
Tower blasted the FDA for its handling of hip implants, charging that the agency has been “asleep at the switch” and has prioritized the interests of device manufacturers over those of patients.
“It is a public health travesty,” Tower said. “I am tired of being the guy who has to look under the rock to find this.”
He said that hip implant patients’ symptoms closely resemble those reported by women implanted with Essure, a contraceptive device containing nickel that was removed from the market last year after patients filed thousands of lawsuits related to its complications.
Madris Tomes, an expert on FDA adverse events data, testified that metal-on-metal hips were by far the most commonly cited product in reports of immune and allergic reactions to devices, according to her analysis of FDA data collected in her proprietary Device Events database.
The next most frequently cited devices, she said, were Essure and other types of hip implants such as metal-and-polymer implants.
Unlike hearings earlier this year on breast implants and vaginal mesh that focused on specific products and regulatory steps, yesterday’s hearing focused on trying to understand and improve the state of scientific knowledge on metal reactions.
As such, it is unlikely to result in the short term in dramatic steps such as banning products from the market.
Experts urged the FDA to make the hearing the beginning of a sustained effort to gather data on the problem of metal reactions, including their differential effects among patients of different gender, age and socioeconomic status.
For example, the substantial majority of adverse responses to metal devices occur among women, who are generally more vulnerable to auto-immune, rheumatic and thyroid disorders.
“There’s a need for more and better pre-market research,” said Diana Zuckerman, the president of the National Center for Health Research. “We need to really have better data on diversity of patients.”