Jan 31, 2024
Whistleblower accuses medical tech giant Medtronic of putting ‘profit before patients’
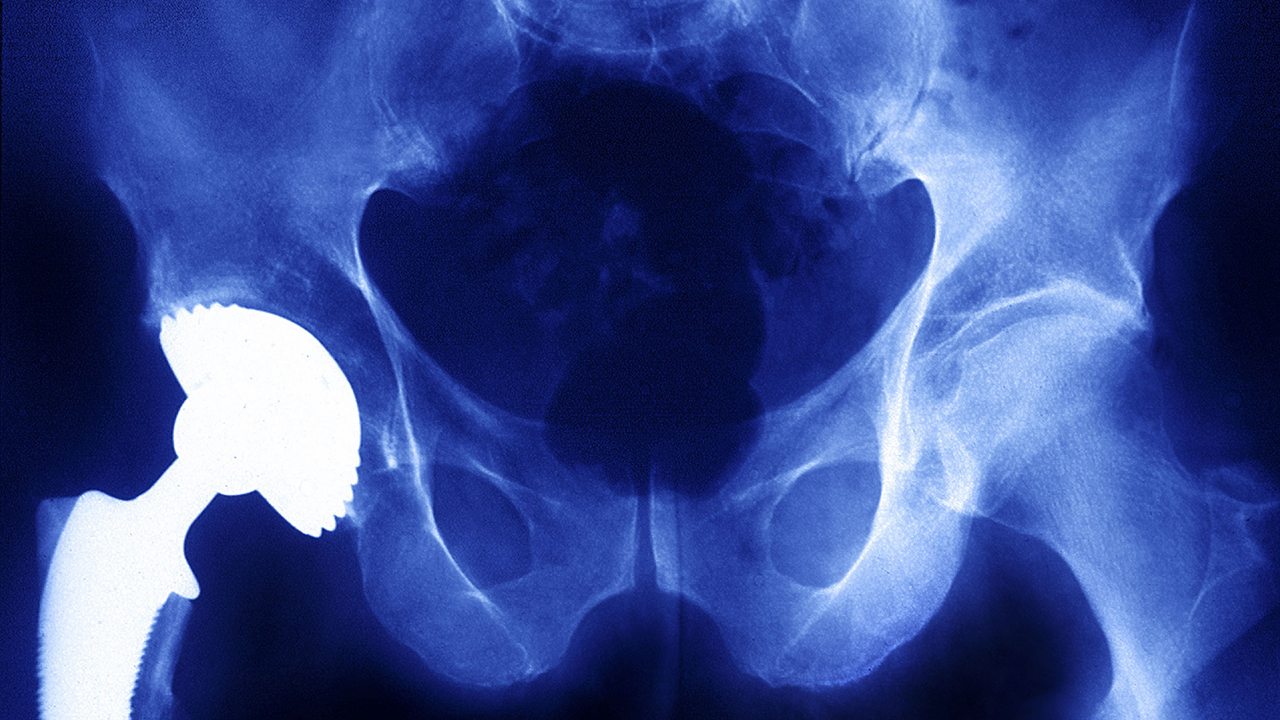
ICIJ’s Implant Files uncovered controversial deals between Medtronic and hospitals around the world. Now, a former U.S. sales representative alleges the company used kickbacks and pressure tactics to make sales, despite patient injuries.