Australia’s medical device regulator has pledged to fast-track safety reforms and improve transparency in a new action plan, but critics of the plan have said that it lacks substance and detail.
The Therapeutic Goods Administration released the 2,600-word Action Plan for Medical Devices in response to “patient concerns” raised by recent media coverage of medical device safety, including the International Consortium of Investigative Journalists’ Implant Files investigation.
ICIJ’s Australian media partners, the ABC and the Australian Financial Review, revealed holes in regulatory systems that allowed potentially harmful products be implanted in unsuspecting patients.
Improvements must be made mandatory and properly resourced. That’s essential for future-proofing the regulation of health products – Associate Professor Wendy Bonython
Australia’s response to the Implant Files investigation joins a growing list of announcements and action, including:
- In France and Canada, bans on certain models of breast implants;
- In Canada and the United States, mandatory or improved disclosure of malfunctions or medical errors, known as “adverse events”;
- In Italy, Germany, the United Kingdom and the Netherlands, proposed registries of medical devices;
- In India, a historic meeting of regulators to discuss a “road map” for device regulation.
In announcing the action plan, the TGA echoed the experience of hundreds of patients, medical safety advocates and doctors who spoke to ICIJ and its media partners in stating that “more can be done” and that “transparency can also be significantly improved.”
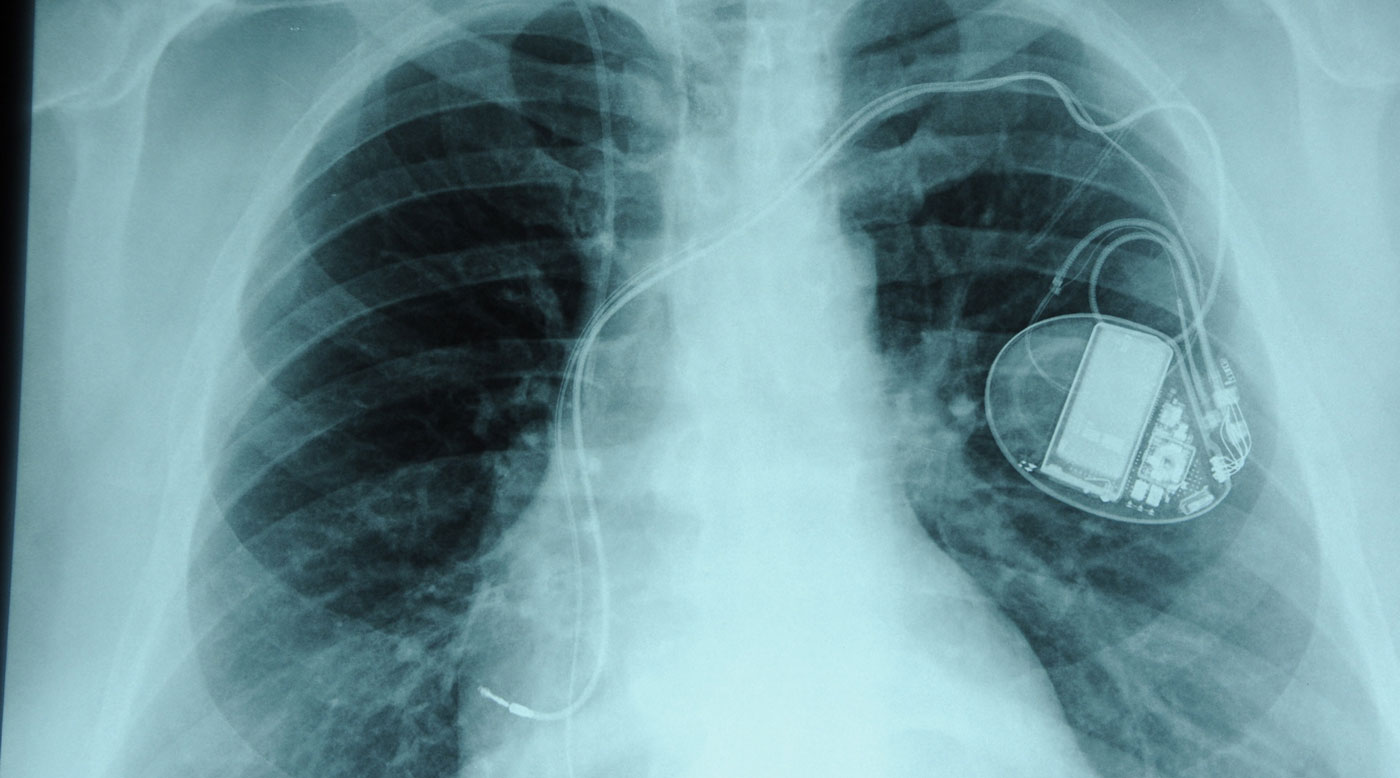
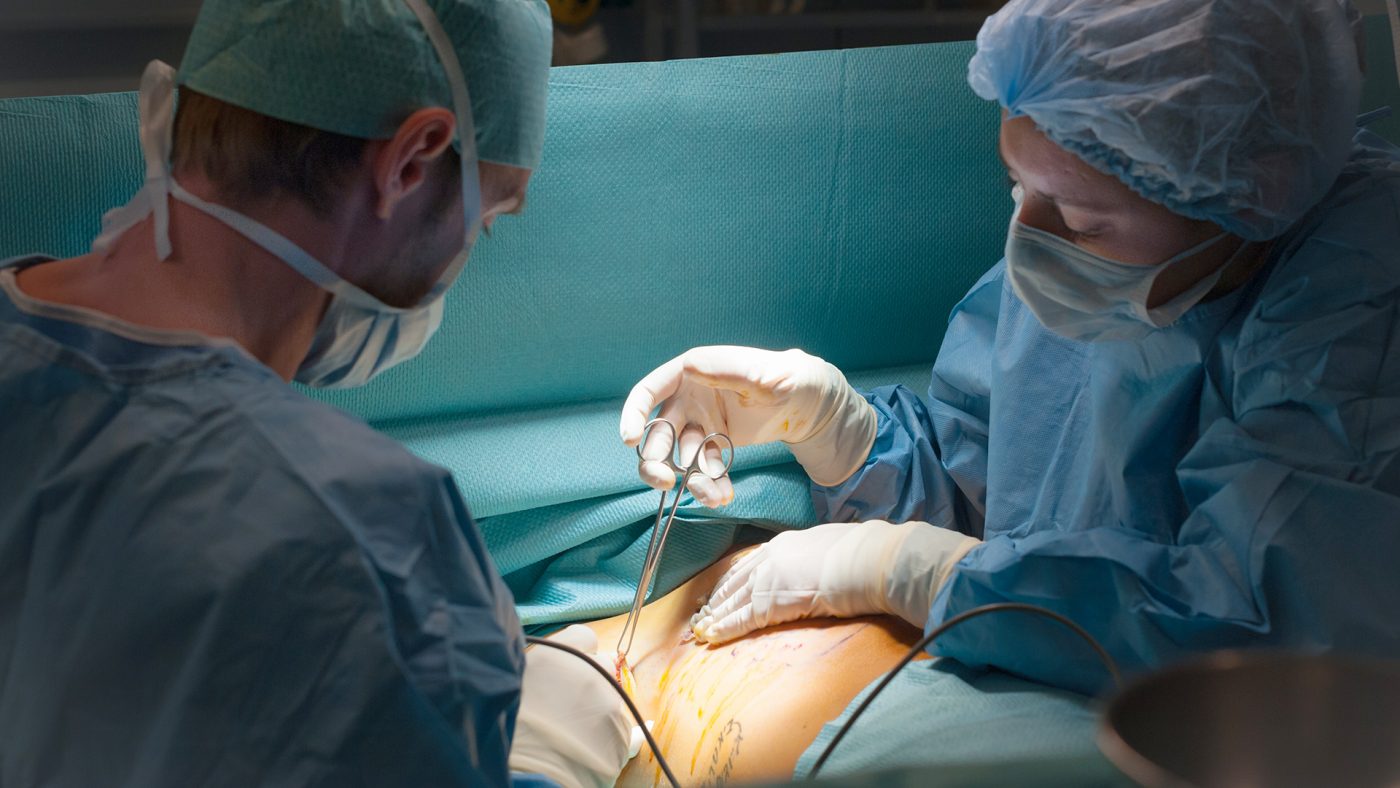
The action plan outlines a raft of proposals, including more inspections of medical device manufacturing sites and stronger powers to recall dangerous devices. The TGA also announced consultations on a device identification system that would allow for better tracking of implanted products and help patients access specific information about the device placed inside their bodies.
Noting “a very low level of consumer awareness” about medical device regulation and challenges searching online data, the TGA will partner with consumer groups, including women’s health advocates, to provide patients with more information and encourage patients to lodge reports when something goes wrong with their device.
The action plan begins with consultation and will require parliamentary approval before any substantive changes can be made.
“We welcome any regulation that will help protect patient safety,” said Chantal Yeaman, founder of the volunteer-run Mesh Awareness Australia.
But Yeaman, who believes the TGA has failed to prosecute or adequately respond to recent breast implant and pelvic mesh scandals, criticized the action plan for being “all plan, no action.”
“It’s an action plan without any substance,” said Yeaman, who is calling for a government-backed commission into Australia’s regulation of medical devices.
Long-time watchers of the TGA also expressed skepticism.
“Despite its name, this is not an action plan,” said Wendy Bonython, an associate professor at the University of Canberra who has previously criticized the TGA for lacking independence and resisting transparency.
Bonython told ICIJ that although the plan “sounds impressive,” it lacks detail, will take years to put in place and requires “significant legislative change.”
“Improvements must be made mandatory and properly resourced,” Bonython said. “That’s essential for future-proofing the regulation of health products as we enter the era of high tech, precision medicine.”