Bayer AG systematically failed to report to U.S. authorities thousands of complaints of injuries allegedly caused by its Essure contraceptive device, experts acting for tens of thousands of women claim in recently unsealed court documents.
The documents, filed on behalf of plaintiffs in consolidated litigation in California, include testimony from experts who conducted a statistical analysis of complaints by Essure patients. They allege that Bayer and its predecessor, Conceptus, reported only a fraction of incidents in which patients said they had potentially been injured by Essure.
Manufacturers are legally required to report injuries linked to their devices to the U.S. Food and Drug Administration.
Dr. Kimber Richter, a witness for the plaintiffs who previously served as Deputy for Medical Affairs at the FDA’s Office of Compliance, testified: “These failures were systemic in that they continued for almost a decade and were not simply isolated events.”
Bayer disputes the claims and dismissed the statistical analysis underpinning them as tainted by flawed methodology. The manufacturer said the disputed complaints were not legally required to be reported.
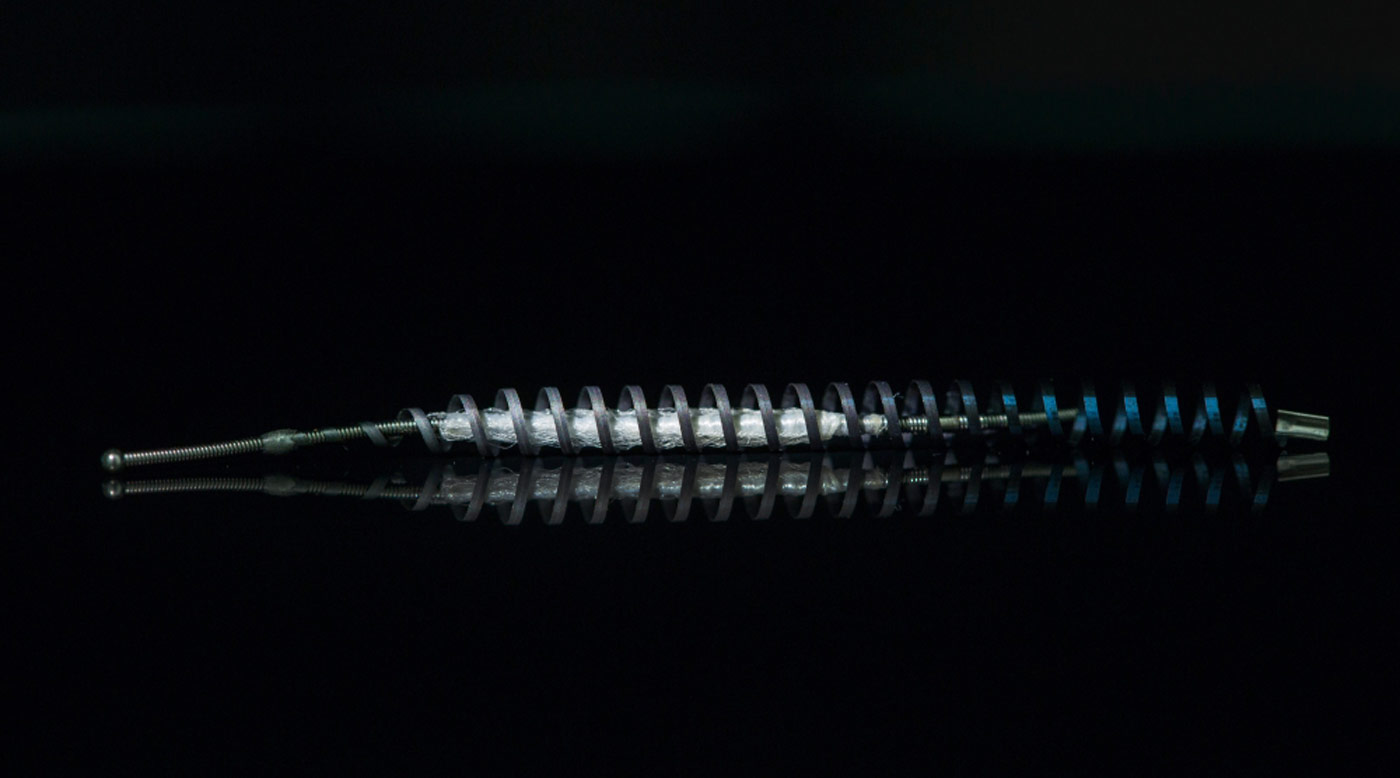
Essure, a contraceptive coil implanted in the fallopian tubes, was first brought to the market in 2002 by Conceptus. Since then, it has been the subject of tens of thousands of complaints from women who have reported symptoms including intense pain, bleeding, organ damage and migration of the device within the body.
More than 27,000 women in California have filed claims against Bayer related to their use of Essure, which have been consolidated in California’s Alameda Superior Court. The cases are now heading to trial after Bayer failed earlier this year to get the claims dismissed.
The analysis of patient complaints conducted as part of the litigation concluded that underreporting by Conceptus, which was acquired by Bayer in 2013, was pervasive and sweeping in scope. Drawn from a sample of over 5,000 complaints filed over more than a decade, the analysis found:
- 24% of complaints should have been reported to the FDA, but only 5.5% were actually reported
- 78% of complaints failed to properly explain Conceptus’ decision of whether or not to report the incident to the FDA
- 89% of complaints were noncompliant in some way with FDA rules for complaint investigation
Bayer strongly disputed the conclusions, saying the analysis was fundamentally flawed due to errors in its methodology. These errors included double-counting of cases and assessments about which cases were reportable that sometimes contradicted the FDA’s own findings for the same incidents, Bayer said.
“The information at issue was prepared by plaintiffs’ paid witnesses in litigation against Bayer who are not statisticians, relies on a flawed and manipulated methodology, contains numerous errors and is at odds with the facts and FDA’s own review of the same data,” Bayer said in a statement to the International Consortium of Investigative Journalists.
Bayer said the FDA had repeatedly inspected its facilities and designated the company as being in “substantial compliance” with its rules for reporting and handling complaints.
“The company continues to stand behind Essure’s safety and efficacy,” Bayer said.
In December 2018, ICIJ reported on how noncompliant manufacturers and weak regulators allowed harm caused by medical devices to be concealed on a massive scale, as part of its global Implant Files investigation.
More than 16,000 complaints about Essure poured into its manufacturer, Conceptus, from 2008 to 2010, but Conceptus only reported 183 incidents to the FDA during that period, ICIJ reported. Not all complaints must be reported to the FDA — for example, cases in which a device malfunctions in a way that does not harm or create the risk of harm for a patient — but many women have alleged that their reports of serious injuries were buried by the company.
After annual reports of suspected injuries linked to Essure spiked from 25 in 2010 to 1,788 in 2014 and 12,564 in 2017, the FDA ordered a sales restriction in 2018. Soon after, Bayer announced it was withdrawing the product from the U.S. market, the last country where it was still sold, a decision it said it made for business reasons. By that time, an estimated 750,000 Essure devices had been sold, according to the Washington Post.
Dr. Joseph Ross, a professor of medicine and public health at the Yale School of Medicine, said the FDA relies on manufacturers to file accurate reports of patient harm to be able to ensure the safety of devices that are already on the market. Device makers are required by law to inform the FDA of deaths, injuries and dangerous malfunctions suspected of being linked to their devices. Reporting by doctors and patients is voluntary.
“Underreporting mutes the potential for the FDA to identify a safety problem,” Ross said. “If the company itself is not reporting, then it just won’t show up.”
Ross is the co-director of Yale’s Collaboration for Research Integrity and Transparency, an initiative that promotes public access to medically significant data in court proceedings. Working with the watchdog group Public Justice, Ross filed a motion in February in the California Essure case calling for court records to be unsealed.
In the months after Ross’ motion, Bayer voluntarily made public hundreds of previously-sealed case documents, including the expert testimonies on its complaint handling. (Public Justice has published the documents here.)
Bayer said it made the documents public as part of the “routine course” of the California litigation, in part due to decreased concerns about proprietary or otherwise confidential information now that Essure is no longer on the market.
Madris Tomes, a former FDA data analyst and the founder of Device Events, a company that tracks medical device adverse events, said that even though Essure is no longer being sold, women are continuing to report injuries associated with the device.
She said that her Device Events database has recorded thousands of incidents linked to Essure in 2020 to date.
“Women still have it implanted,” Tomes said. “These adverse events reports haven’t slowed down.”
Correction, Aug. 6, 2020: An earlier version of this story incorrectly referred to the California cases against Essure as a class action lawsuit. In fact, the cases have been consolidated in mass tort litigation, in which the pretrial proceedings are coordinated but cases remain distinct.