The Pentagon has announced a new program to better oversee human cadaver tissue used in Defense Department hospitals around the world and is investigating allegations that some tissue-based medical implants provided to service members may have been obtained improperly, military officials said Wednesday.
At the same time, Congressional investigators say they are looking into government contracts between the Department of Veterans Affairs and RTI Biologics, a Florida-based manufacturer of medical implants made from human bones, skin, ligaments and other tissues. RTI is one of the world’s largest players in the billion-dollar human tissue industry – processing a quarter of all material recovered from cadavers in the United States.
The International Consortium of Investigative Journalists reported in July that RTI had obtained tissues from suppliers in the U.S. and the Ukraine that have been investigated for allegedly forging documents or bullying families into signing donor consent forms.
“We are currently in the process of determining if our Military Treatment Facilities – administered by the Army, Navy, and Air Force respectively – have conducted business with RTI or its subsidiary, Tutogen,” Defense spokeswoman Cynthia Smith said in a prepared statement.
Smith said the Pentagon’s new human tissue oversight program applies to 59 hospitals and 362 clinics run by the Defense Department in the U.S. and around the world. She said the Defense Department review of government contracts with RTI was directly prompted by the ICIJ’s findings.
In a July 26 directive made public this week, Assistant Secretary of Defense Jonathan Woodson noted that the use of human tissue implants has grown rapidly in recent years and ordered Army, Navy and Air Force health facilities using human tissue to update their existing policies. He directed medical staff to thoroughly investigate any infections that might be linked to tainted tissue. He said the armed services should obtain tissue from qualified suppliers and hold them accountable for any problems.
Woodson said the new program must have recordkeeping that ensures “tissue traceability from the supplier to the patient” and that any recalls stemming from infections must have “clear traceability” so other affected patients can be contacted. ICIJ’s investigation found that human tissue products often lack bar codes or other traceable markings, making it difficult for health officials to alert patients who may have come into contact with tainted tissue.
U.S. officials are looking into the safety of human tissue products implanted into active duty service members and veterans, Miltary.com reported this week.
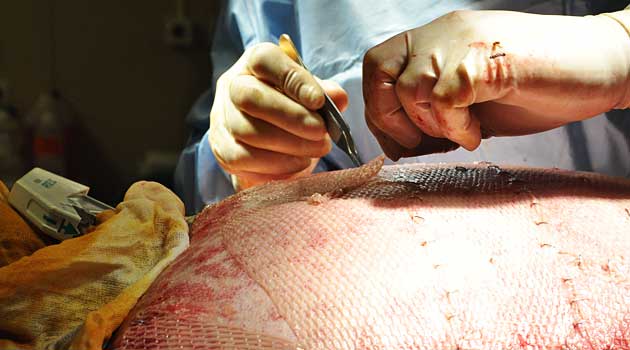
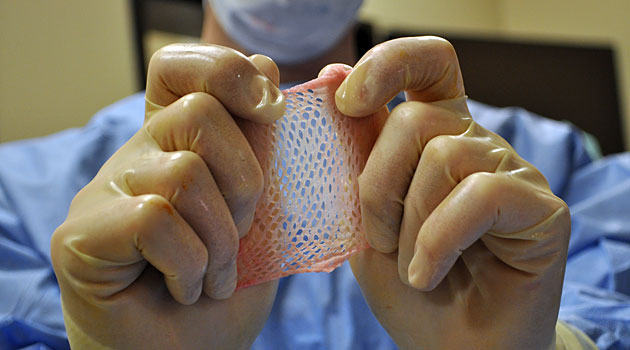
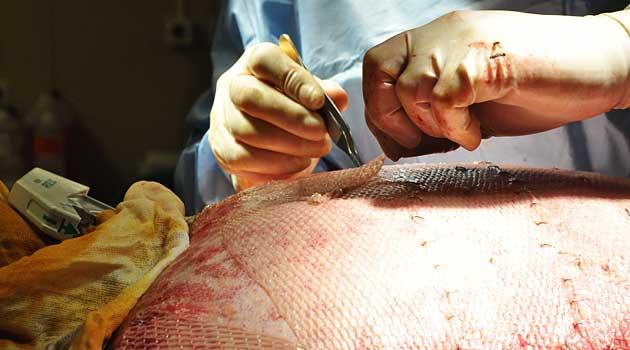
The government probes come amid increasing scrutiny of the global trade in human tissue, which harvests parts from the dead and recycles them into dental implants, skin grants, cornea transplants and other products.
A four-part series last July by the ICIJ found much of the international market was loosely regulated, and that bones, skin, ligaments and other human tissue may have come from unwilling donors in the Ukraine and elsewhere.
The articles raised questions about how tissue is harvested and how well it is tracked. Some experts worry inadequate safeguards increase the danger that diseased tissues could infect transplant recipients with hepatitis, HIV and other pathogens.
One story in the series examined RTI’s relationship with Michael Mastromarino, the leader of a U.S.-based human tissue trafficking ring that stole parts from grandmothers, electrical engineers, and factory workers. Mastromarino, who is now serving a lengthy prison sentence for fraud and theft, sold tissues to RTI from 2002 to 2005.
In August, Military.com cited ICIJ’s investigation in a news article that revealed RTI has supplied human tissue-based products to Department of Veterans Affairs and Defense Department hospitals around the world.
The story said the VA and Defense Department have bought from RTI since about 2004. Authorities have not accused RTI or Tutogen of wrongdoing.
An RTI spokeswoman told Military.com that the company “has a long history as a responsible steward of the gift of donation and delivering safe tissue implants for patients in need.”
“RTI fully complies with comprehensive regulations, both from U.S. regulatory authorities and those of other countries, that govern each and every activity performed by tissue banks,” she said. RTI declined comment for this story.
Military.com’s Bryant Jordan reported that the House Committee on Veterans Affairs has also begun to investigate issues of safety and traceability. A source told Jordan that committee staffers are reviewing RTI contracts with the Department of Veterans Affairs hospital system and “looking at whether the VA used any contaminated materials and whether anyone was tracking it.”
Committee chairman Bill Johnson (R-OH) confirmed Wednesday that his office is looking into the contracts. “Once the Committee has completed its investigation, we will be taking appropriate action to ensure that all biologics purchased by VA are not only in line with the law, but also that only safe biologics are being used on our veterans,” he said.