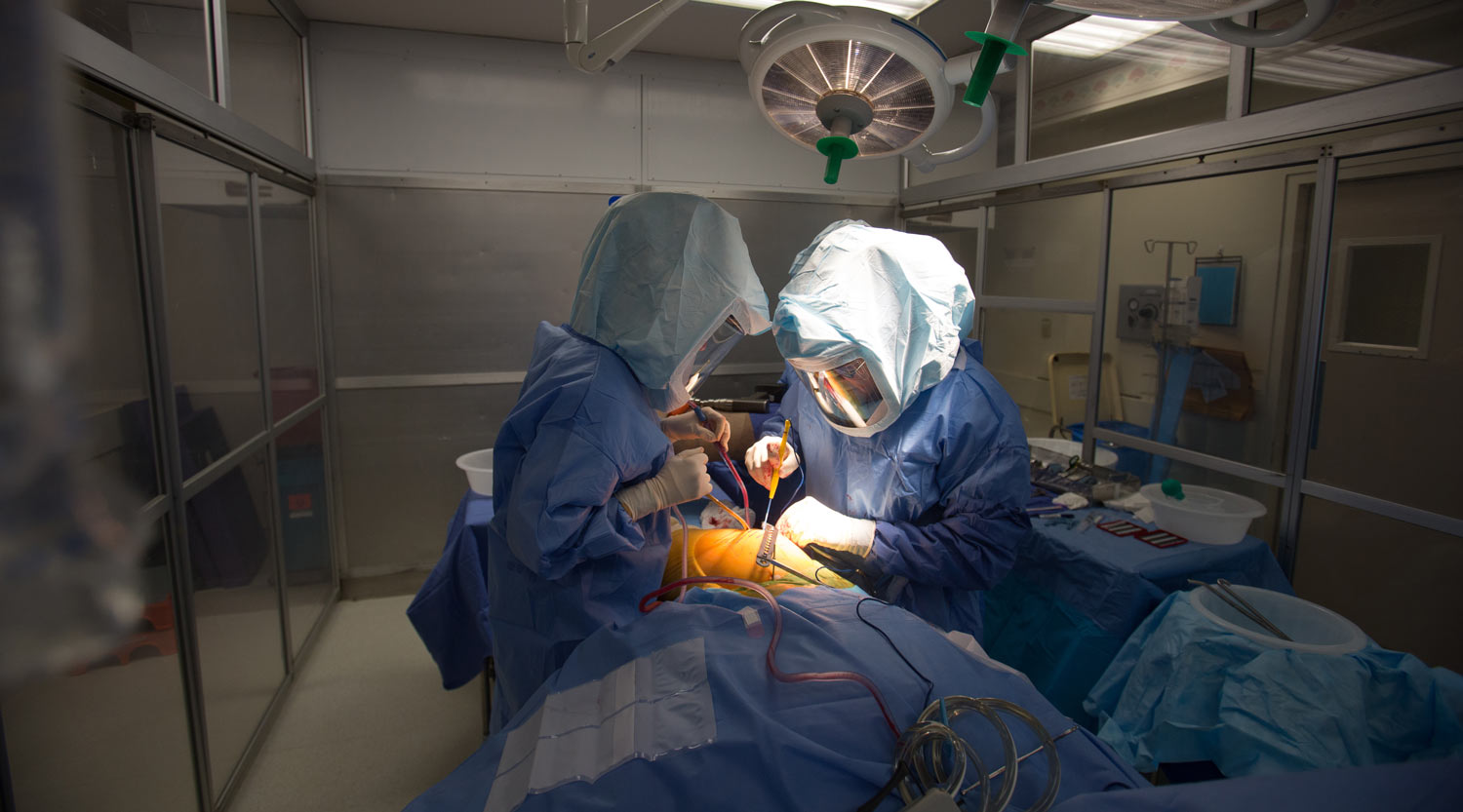
Government regulators and consumer advocates from Europe are calling for stepped up measures to protect patient safety, following the latest investigation by the International Consortium of Investigative Journalists into how lax medical device regulation allowed flawed devices to come to market.
German health minister Jens Spahn announced an overhaul of that country’s registry of medical devices in an interview with ICIJ partner newspaper Süddeutsche Zeitung.
“We will build an institution which is independent from industry, where all implanted devices need to be registered,” Spahn said, adding that the register will help inform patients about problems with implants and provide advice on the lifespan of devices.
“We are going to tighten the control in Denmark,” health minister Ellen Trane Nørby told ICIJ media partner, Danish Broadcasting Corporation, adding that her ruling party will increase funds for the Danish Medicine Agency.
She noted Europe-wide safety rules that will come into effect in 2020 but that have been criticized for keeping patients and doctors in the dark about implant safety but added that she couldn’t “rule out that we have to tighten the rules even further.”
Italy’s health minister, Giulia Grillo, thanked the Italian TV show and ICIJ media partner Report for its part in the investigation and announced an expansion of the existing medical devices registry. According to the new proposed rule, regional health bodies will be mandated to register all medical devices.
“Citizens must know that there will be no more tolerance on the opacity of the system,” Grillo said.
Spain’s College of Medicine called for a review of European regulations, according to ICIJ media partner La Sexta.
Spain’s nonprofit consumer advocacy group, Facua, criticized “the lack of notification” when a medical device is withdrawn. Implant patients “receive virtually no information” when a recall alert is issued, Facua said in a statement.
The parliamentary leader of France’s communist party, André Chassaigne, requested the creation of a committee to probe “the shortcomings in the control of medical implants,” according to a letter obtained by ICIJ media partner Le Monde.
We will certainly take advantage of this moment to see how we can still do better with all players in the sector.
In the United Kingdom, the Royal College of Surgeons said the investigation shows “urgent” and “drastic” changes are needed to protect patient safety, and called for a new register to be set up to track every device in every patient.
In Slovenia, ICIJ media partner, Oštro, reported that the national regulator announced for the first time its intention to publish safety notices online about medical devices.
Elsewhere, government agencies took advantage of the Implant Files to defend their safety records and to highlight the work they have already done.
“We will certainly take advantage of this moment to see how we can still do better with all players in the sector,” said Xavier De Cuyper, the general administrator of Belgium’s medical agency.
Continent-wide, the Greens Group at the European Parliament called for an “in-depth debate” in response to revelations of flaws in the way implants are approved.