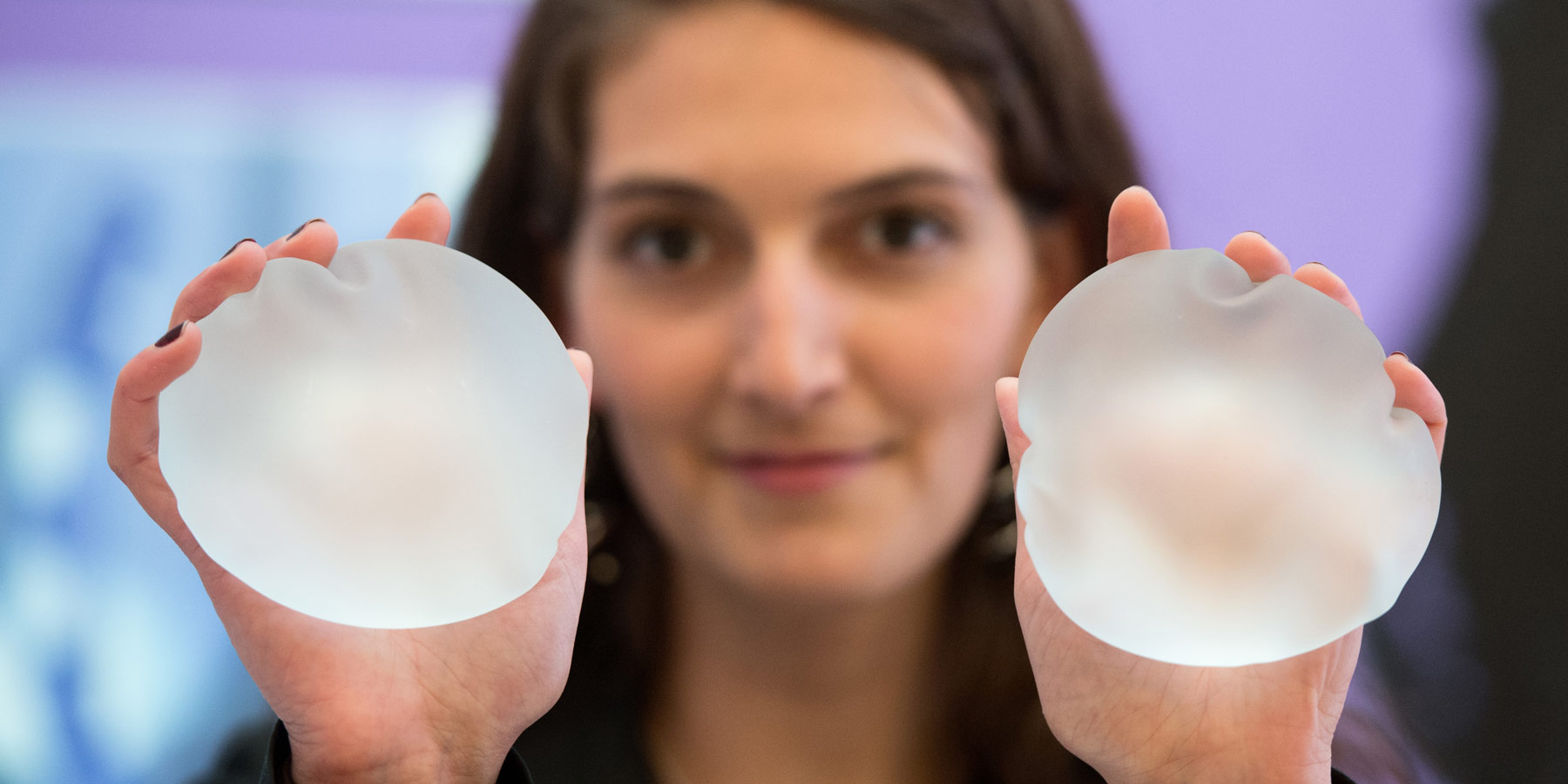
Breast implant safety is a subject of growing international concern, and at the center of the controversy is a rare form of cancer that occurs most often in implants with rough surfaces known as textured implants.
The International Consortium of Investigative Journalists revealed the ongoing health problems plaguing women with breast implants as part of its global Implant Files investigation in November 2018, and has covered breast implant safety extensively in the aftermath. In the wake of the investigation, health authorities from France to Brazil to the United States recently announced initiatives to better protect patient health.
With public concerns increasing and major decisions on breast implant safety expected soon from regulators in several countries, ICIJ answers the key questions about textured implants and their safety.
What is a textured breast implant?
Textured breast implants have a rough surface that is sometimes compared to sandpaper. Unlike smooth-surfaced implants, their surface adheres to the tissue that surrounds them, preventing them from moving around within the implant pocket created by the surgeon. This is especially beneficial for implants that are not round but tear-drop shaped, because movement or rotation of these implants would cause the patient’s chest to appear misshapen.
What is the connection between textured breast implants and cancer?
Studies have shown that patients with textured implants face a higher risk of a rare form of cancer called breast implant associated anaplastic large cell lymphoma (BIA ALCL). BIA ALCL is not a breast cancer but a cancer of the immune system. Plastic surgeons have identified at least 732 cases of BIA ALCL worldwide, as of June 2019. The FDA estimates the risk of BIA ALCL among patients with textured implants as between 1 in 3,817 and 1 in 30,000, but newer data from Australia has placed the risk as high as 1 in 1,000.
While the vast majority of BIA ALCL cases occur in patients with textured implants, the FDA has identified at least 24 in patients with smooth-surfaced implants.

Why are health authorities investigating textured implants now?
Regulators have recognized BIA ALCL since 2011, when the U.S. Food and Drug Administration issued its first report on the disease. But aside from acknowledging BIA ALCL’s existence, health authorities did not take any action until a series of events in late 2018.
In November 2018, ICIJ and its media partners around the world published the Implant Files, which included an in-depth investigation of breast implant safety and the risks of ALCL. The following month, the European certification for textured breast implants produced by Allergan expired. Rather than renewing the certification for another five years, which usually occurs routinely, a European oversight body declined to certify the products. This decision set off a chain reaction among regulators around the world as they re-examined the safety of textured breast implants.
Are textured implants still allowed on the market?
In recent months regulators have restricted certain brands of textured implants. In April, French authorities banned macro-textured and polyurethane implants, two categories of textured implants associated with higher risks of ALCL. The prohibition came after textured implants produced by leading manufacturer Allergan were suspended from the European and Brazilian markets in December 2018 after losing their European certification. This February, a French government inquiry recommended that one brand of textured implants, Allergan Biocell, should be permanently banned.
U.S. and Canadian regulators are now investigating breast implant safety but neither has imposed restrictions while their inquiries are underway. Textured implants produced by manufacturers other than Allergan remain on the market worldwide, with the exception of the brands banned in Europe.
Why do textured implants have a higher risk of BIA ALCL?

The cause of BIA ALCL is still not fully understood. Some plastic surgeons believe that it originates with a bacterial infection in the area surrounding the breast implants. They think the crevassed surface and higher surface area of textured implants may provide more opportunity for bacteria to lodge than a smooth-surfaced implant.
Some experts have proposed classifying textured implants on a scale from 1 to 4, with higher numbers indicating greater surface and roughness, and therefore greater risk of BIA ALCL. The differences between implant models can be dramatic. According to one forthcoming study, Silimed polyurethane textured implants pose a 23- times higher risk of ALCL, and Allergan Biocell textured implants pose a 16- times higher risk of ALCL, than lower surface area Siltex textured implants by Johnson & Johnson. Based on data from Australia and New Zealand, the study will appear in the May issue of the journal Plastic and Reconstructive Surgery.
The French government inquiry that issued its report in February called for a European-wide system of classification to be applied to textured implants.
What will happen next?
Various health authorities are investigating the safety of textured implants and will decide whether to impose restrictions or additional regulations.
France has now banned macro-textured and polyurethane implants. That decision is likely to have wider repercussions in Europe. The FDA is holding a public hearing of its General and Plastic Surgery Devices panel to address breast implant safety on March 25-26, with BIA-ALCL as a key issue on the agenda. The panel will issue recommendations for next steps, and public hearings are sometimes a preliminary phase before the FDA announces new policies. The agency’s decision will likely influence regulators in Canada, which is also investigating textured implants and ALCL, and elsewhere in the world.
Update, April 3, 2016: This story was updated following the decision by French authorities to ban a number of textured breast implants that have been associated with a higher risk of cancer.
Update, July 11, 2019: This story was updated with the latest figures on the number BIA-ALCL confirmed worldwide.