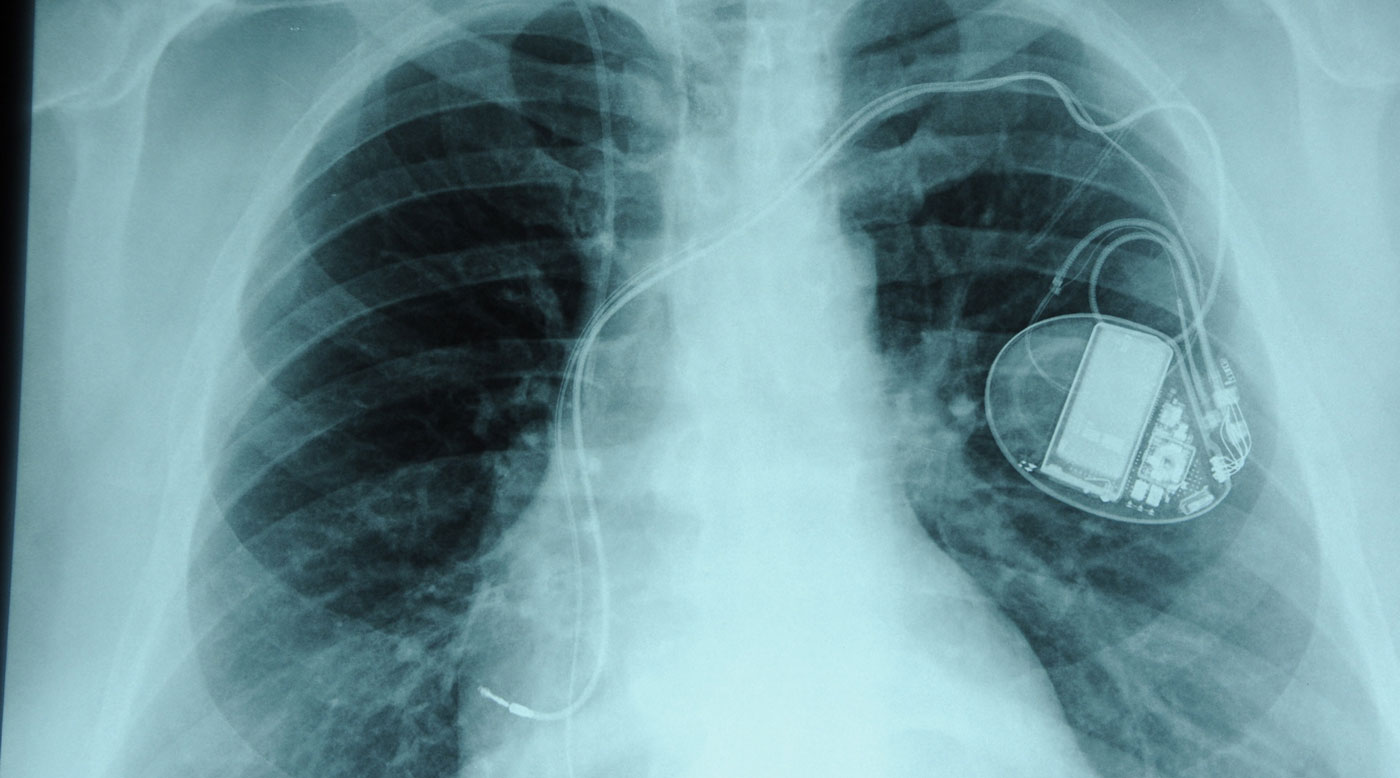
Health authorities across the globe have failed to protect millions of patients from poorly tested implants that can sicken, maim — and sometimes kill — the people they were designed to help, new global investigation the Implant Files has found.
The International Consortium of Investigative Journalists, which previously produced the Panama and Paradise Papers investigations, partnered with more than 250 journalists in 36 countries to examine how devices are tested, approved, marketed and monitored.
Medical devices provide clear, even life-changing benefits in most cases, radically improving health and sometimes saving lives. But ICIJ’s reporting raises questions about whether the device industry is needlessly putting patients at risk of serious harm in its quest for profits.
The investigation has found that even complex, high-risk implants are held to a lower safety testing standard than most new drugs. Flawed devices linger on the global market unrecalled as injuries mount. Under a free-for-all system, device companies pull implants in some countries while continuing to sell them in others.
Anchoring the probe is an analysis of more than 8 million device-related records, including death and injury reports and recalls. To increase transparency and to provide information around the world where such information is scarce, we have built the world’s only publicly searchable global database of recalls and safety warnings. The International Medical Devices Database includes 70,000 records from 11 countries, and will be updated with data from more jurisdictions in the coming weeks.
Among the findings of this year-long investigation into the regulation of devices that touch billions of lives:
- More than 1.7 million injuries and nearly 83,000 deaths suspected of being linked to medical devices over 10 years, and reported to the U.S. alone
- Governments in dozens of countries in Africa, Asia and South America don’t regulate medical devices at all, instead placing their trust in approvals by European authorities or the U.S. Food and Drug Administration.
- Manufacturers have paid at least $1.6 billion since 2008 to settle charges of corruption, fraud and other violations with regulators in the U.S. and other countries, according to an ICIJ review of data from the U.S. Justice Department and the Securities and Exchange Commission.
- The global team made more than 1,500 Freedom of Information requests for access to government-held records, and spoke to hundreds of patients around the world.
- Doctors and manufacturers often fail to report adverse events, and when they do, the information can be unverified and incomplete. Over large swaths of the planet, health authorities refuse to disclose information about harm to the public — or just never collect it in the first place.
- Using a machine learning algorithm to search millions of reports, ICIJ found 2,100 cases where people died, but their deaths were classified as device malfunctions or injuries only. Of these, 220 reports indicated that devices may have caused or contributed to the deaths.
- Whether a product is recalled or restricted may depend on where you live. Experts told ICIJ that governments should publicly issue recall and safety alert notices so patients and doctors are aware of problems. An ICIJ analysis found that some governments post notices frequently, and some almost never do. Health regulators in Mexico have only shared information on two issued recalls ever. In the U.S., the FDA has published more than 26,000 recalls in the last decade.
- The EU has borne the brunt of many of the world’s worst implant scandals, unnecessarily resulting in patients scarred, injured or in permanent pain, or even dead. Leading surgeons, regulators, lawyers and patient advocates have said Europe’s approach treats its citizens “like guinea pigs.”
- Responses from regulators in 19 European countries — responsible for the safety of more than 85 percent of EU citizens — to an ICIJ request for the raw numbers of reported malfunctions, injuries and deaths show a steep rise in incident reports over recent years.
The Implant Files investigation builds on reporting in the Netherlands by Jet Schouten, an investigative journalist for Dutch Public Broadcasting, which was part of the international team that included 58 media partners.

Even before our first stories were published, after receiving questions from ICIJ, regulators in the U.S. and Europe announced policy changes and renewed efforts to protect patient safety
ICIJ and its partners will release more findings in the coming days, including reporting on the hidden dangers of breast implants, a new heart valve device that may be risky for younger patients, and the often excruciating experiences of people living with broken medical implants that can’t be removed from their bodies.
ICIJ and its partners welcome more information from whistleblowers, insiders and patients to help us continue our reporting and expand our data. Please contact us via this form or any of these secure platforms.
For general inquiries, please email contact@icij.org. For data inquiries or requests, please email data@icij.org. Media can contact ICIJ at +1 202 808 3310.