A fresh U.S. Food and Drug Administration probe into adverse patient reactions to materials in medical devices, including breast implants, metal-on-metal hips and the birth control coil Essure, has been announced.
The FDA says it will hold a public hearing this fall into nitinol as part of the investigation into inflammatory and immune responses in patients; it will task its own scientists to conduct research on medical implant materials; and it will review published studies.
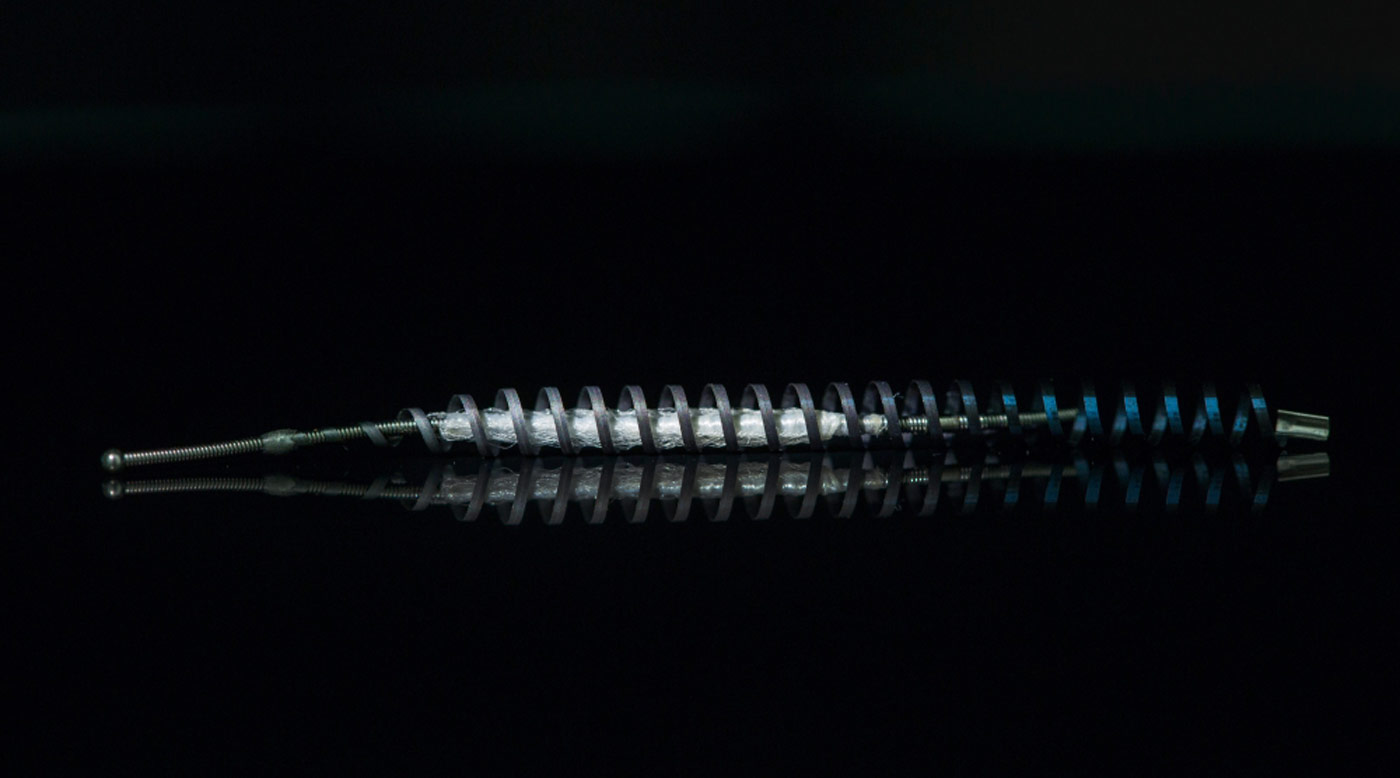
Nitinol is an alloy containing nickel that is used in Essure. Many patients have reported allergic reactions to Essure and the U.S. Centers for Disease Control have estimated that between 10 and 20 percent of the population is allergic to nickel.
The agency will also consider patient responses to the silicone contained in breast implants in a previously announced hearing on breast implant safety due to take place on March 25-26.
“We believe the current evidence, although limited, suggests some individuals may be predisposed to develop an immune/inflammatory reaction when exposed to select materials,” the FDA said in its statement.
Last fall, the International Consortium of Investigative Journalists reported extensively on breast implants, Essure and metal-on-metal hips as part of the global Implant Files investigation of the medical device industry and its overseers. ICIJ’s coverage examined regulators’ refusal to recognize adverse reactions to these devices among patients, including breast implant illness, an ailment reported by many breast implant patients characterized by chronic fatigue, muscle pain and cognitive difficulties.
The announcement marks a shift in the agency’s approach toward breast implant illness in just the last six months.
On Sept. 14, 2018, after a study by the respected MD Anderson Cancer Center found elevated risks of autoimmune disease among patients with breast implants, the FDA issued a statement indicating that agency officials “respectfully disagree with the authors’ conclusions.”
In its March 15 announcement, the agency appeared more open to the possibility that breast implants could increase the risks of fatigue, muscle pain and cognitive issues among some patients.
“While the FDA doesn’t have definitive evidence suggesting breast implants are associated with these conditions, we’re looking to gain a fuller understanding of this issue to communicate risk, minimize harm and help in the treatment of affected patients,” it stated.
The FDA is also increasing its scrutiny of nitinol. Last August, a law firm in Australia launched a class action lawsuit on behalf of patients alleging that their Essure implants corroded and caused them to suffer nickel poisoning. The suit is expected to be filed in May, said a spokeswoman for the law firm, Slater and Gordon.
Essure was withdrawn from the market worldwide at the end of 2018.
However, the FDA noted that nitinol is used in other implants such as cardiovascular stents and guidewires, and said that it will issue draft guidelines in the coming months for the use of nitinol in medical devices.
Clarification, March 21, 2019: ICIJ has clarified this story to reflect that the Essure lawsuit in Australia was launched in August 2018 but has not yet been filed.